begin the titration Switch to graphing method in order to notice the titration curve improve in actual time.
The purpose at which the response is total is referred to as the equivalence issue, usually signaled by a coloration modify or other observable adjust, and the process of titration entails many types and different ways of titration:
All other points being equal, the toughness of a weak acid raises if we area it inside of a solvent that is much more essential than drinking water, and the toughness of a weak base increases if we position it in a solvent that is additional acidic than drinking water. In some instances, nonetheless, the other impact is observed. For instance, the p
Any Alternative that contains comparable amounts of a weak acid, HA, and its conjugate weak base, A–, is often a buffer. As we figured out in Chapter 6, we can work out the pH of the buffer utilizing the Henderson–Hasselbalch equation.
For acid-base titration, a contemporary lab will often keep an eye on titration which has a pH meter which is interfaced to a computer, so that you'll be in a position to plot the pH or other Bodily quantities vs . the quantity that is certainly included.
A conservation of mass on nitrogen calls for that every mole of NO2 makes one particular mole of HNO3; Therefore, the mass of NO2 inside the sample is
As well as a quantitative Evaluation along with a qualitative Evaluation, we can also use an acid–base titration to characterize the chemical and physical Homes of make any difference.
Complexometric titration steps steel ion concentrations by using a chelating agent to kind stable complexes with metallic ions in an answer. The level of chelating agent necessary establishes the steel ion concentration.
This is done for factors that may type insoluble salts within an aqueous Option. It includes the separation of ions with the compound in the shape of precipitates. The subsequent tend to be the ways involved with the procedure.
A useful indicator has a robust color that modifications swiftly close to its pKa. These features are fascinating so only a small amount of an indicator is required. If a great deal of indicator is utilised, the indicator will impact the ultimate pH, decreasing the accuracy with the experiment.
Be sure you increase enough drinking water to submerge the pH probe and go ahead and take dilution outcome of this drinking water under consideration when deciding the Original concentration of your acid.
would not continue to an important extent for the reason that CH3COO– is really a more robust base than H2O and H3O+ is really a more powerful acid than CH3COOH. If we location acetic acid within a solvent That may be a stronger base than h2o, such as ammonia, then the response
Calculate the pH of your titration Option once the addition read more of the read more following volumes of NaOH titrant:
Which means you find the equivalent position over the titration curve and read the value on the curve at 50 % of that volume. Due to this you'll want to gather knowledge half way along the curve (pink circle).
 Alfonso Ribeiro Then & Now!
Alfonso Ribeiro Then & Now!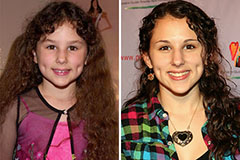 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Keshia Knight Pulliam Then & Now!
Keshia Knight Pulliam Then & Now! Freddie Prinze Jr. Then & Now!
Freddie Prinze Jr. Then & Now! Elisabeth Shue Then & Now!
Elisabeth Shue Then & Now!